Pregnancy in myeloproliferative neoplasms (MPN) is commonly associated with maternal and foetal complications due to increased risks of thromboembolic and haemorrhagic events and placental dysfunction. The incidence of UK pregnancies in MPN is estimated as 3·2/100 000 maternities per year (Alimam, S., et al 2016, Br J Haematol 2016; 175:31-36; Robinson et al, Br J Haematol 2020;189:625-34). Here we present pregnancy outcomes of MPN patients attending University College London Hospital (UCLH), a large UK tertiary referral centre, between 2015 and 2023.
Twenty-three patients were identified with a median age at MPN diagnosis of 33 years (range (r), 13 - 46). The majority of patients (21/23, 91.3%) had a diagnosis of ET or triple negative (TN) thrombocytosis and 8.7% (2/23) a diagnosis of polycythaemia vera (PV). In 61.4% of pregnancies, diagnosis was known at conception. Regarding mutational status, JAK2V617F was present in 10/23 (43.5%) patients, CALR in 5/23 (21.7%) and MPL in 1/23 (4.3%). The remaining 7/23 (30.4%) patients were TN. Median VAF for JAK2V617F and CALR were 15.8% (r, 21.1 - 33%) and 25.9% (r, 5.7 - 46%), respectively.
The cohort collectively experienced 57 pregnancies, 38 of which were managed at UCLH. Focusing on pregnancies managed at UCLH, median age at pregnancy was 34.5 years (r, 22 - 47). Conception was spontaneous in 31/38 (81.6%) pregnancies. Six (15.8%) patients required assisted conception and had a higher median age of 38.5 years (r, 35 - 47). Where gestation at delivery was known, 92.6% (25/31) of live births delivered at term (>37 weeks). Caesarean rate was significant at 41.9% (13/31), of which 61.5% were emergency sections. The live birth rate of the 57 overall pregnancies was 76.9% and 88.6% in the 38 managed at UCLH.
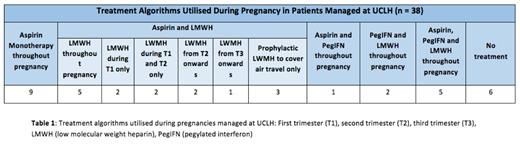
At UCLH, we adopt an individualised approach to managing pregnant women with MPNs, working alongside obstetricians and obstetric haematology colleagues. Consequently, as shown in Table 1, there were bespoke regimens for individual patients. Low dose aspirin was prescribed throughout pregnancy in the majority (78.9%, 30/38) of pregnancies. In 18.4% (7/38), cytoreduction was established prior to pregnancy. In 42.9% (3/7), pegylated interferon (PegIFN) was commenced to optimise counts prior to pregnancy and in 42.9% (3/7) as long term count control. One patient was cytoreduced with hydroxycarbamide prior to pregnancy and subsequently switched to PegIFN. Low molecular weight heparin (LMWH) was administered at some point during pregnancy in 60.9% (14/23) of patients and 57.9% (22/38) of pregnancies. 65.8% (25/38) underwent monitoring with uterine artery (UA) dopplers. Median gestation for UA assessment was 20 weeks (r, 19 - 28). UA dopplers were reported as abnormal in 16% (4/25). In two patients, this was due to a raised combined pulsatility index, UA notching in one and was unspecified in one patient.
Nine (39.1%) patients experienced foetal loss, with 12 miscarriages recorded across the total 57 pregnancies and 4 miscarriages in the 38 pregnancies managed at UCLH. Median maternal age was 31.5 (r, 24 - 42) and median gestation was 8 weeks (r, 5 - 24). Six (66.7%) patients experiencing foetal loss had a diagnosis of ET, 2 (22.2%) PV and 1 (11.1%) TN thrombocytosis. Three miscarriages occurred in one patient, who demonstrated a concurrent heterozygous prothrombin G20210A mutation in addition to ET.
Eight (34.8%, 8/23) patients collectively experienced 11 haemorrhagic events. The majority (72.7%) were post-partum haemorrhage (PPH), 37.5% of which were classified as major or severe. Three (27.3%) bleeding events occurred whilst on aspirin monotherapy, 3 (27.3%) on combined LMWH and aspirin, 1 (9.1%) on LWMH alone and 1 (9.1%) on LMWH and PIFN. With regards to thrombosis, one patient with undiagnosed ET had a cerebral venous sinus thrombosis at 11-weeks gestation. A total of 2/23 (8.7%) patients' pregnancies resulted in hypertensive complications and pre-eclampsia, both resulting in delivery with caesarean section at 34 and 35 weeks.
Although the live birth rate is encouraging, our data highlights that patients with MPN remain at increased risk of miscarriage, early delivery and haemorrhagic events. There remain inconsistencies in the management of pregnancy in MPN and, although we adopt an individualised patient-centred approach, there is an unmet need for further study and development of evidence based treatment algorithms.
Disclosures
Lambert:Blueprint: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Novartis: Honoraria; Kite-Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. McLornan:Abbvie: Honoraria; Jazz Pharma: Honoraria; Imago Biosciences: Research Funding; Novartis: Honoraria; UK ALL RIC TRIAL - DSM board: Other: participation on a data safety monitoring board or advisory board; EBMT Scientific Council Member: Other: Chair of EBMT CMWP. Wilson:Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal